Services
Electronics
We offer standard-compliant hard and software development for simple and more complex mechatronic systems
Prototyping
We create functional models and prototypes according to your ideas using generative manufacturing and as well as CNC-Drilling. Together with our partners we accompany you with every step to series production
Project coordination and project management
In the interdisciplinary field of medical technologies communication between the development partners is the crucial part of accomplishing a well-rounded project
Consulting and approval
We consult you regarding approval, quality and risk management and interoperability (IEEE 11073)
Integration services
Via adapter or as a partner for new or further developments of your product lines — we integrate your medical product according to the open IEEE 11073 SDC standard into the OR device network of the future
Software development
Whether embedded or standalone software: the purpose determines whether software is to be classified as an (independent) medical device.
We support you from there onwards and accompany you through all the steps of development. You will be involved in our development process, which is preferably agile. For us, agil means first of all: requirements are always welcome, at any time. And secondly, we deliver results that you can check at any time — even during development.
In addition, we develop test driven. Writing many automated tests serves the stability and good architectural design that are essential for a medical device.
The standards relevant for software development, such as IEC 62304, must be observed in every development process. For this reason, software development ends with documentation that is compliant to standards, which we produce during development.
Development of electronics
Your company wants to develop, approve or produce a medical product? Thanks to our many years of experience, we can support you in ever phase of the development process. We find the right step in the process to accompany you from the idea to approval, if desired. Our compact multidiciplinary team supports you finding a fast, cost-efficiant and high quality solution.
Prototyping
Our large-volume generative manufacturing system from German Reprap and our 3‑axis CNC milling machine from Isel with modern high-frequency spindle ensure the fast production of high-quality functional samples. Our network of partners in the region includes computer-aided metal part machining (CNC), printed circuit board manufacturers and generative manufacturing technologies companies to produce first-class prototypes for your product. The assembly of mechanical assemblies and electronic circuits can be carried out on site. We are looking forward to turning your concept into reality.
Project coordination and management
In addition to the development of mechanical and electrical components, software technology in particular has become increasingly important in recent years. Due to the digitization and networking of the different systems, a large number of other disciplines have been brought on the agenda, each of which can only be fulfilled by highly specialized IT providers.
Due to the high degree of specialization of the development service providers in their individual core competences, however, it is increasingly necessary that the development of complex medical technology systems can only be carried out in cooperation.
In addition to traditional engineering offices, this includes specialized software providers supported by university institutes and clinical partners. Experienced project coordination is crucial for these heterogeneous consortia to work together effectively. SurgiTAIX has 15 years of experience in coordinating research projects as well as industrial projects and speaks the “languages” of the various cooperation partners.
Consulting: Quality and risk management service providers
Quality and risk management are two concepts that are inherently linked to medical devices. In accordance with the Medical Devices Act, every manufacturer of medical devices is obliged to provide technical documentation for each of its products. This documentation must be prepared in accordance with a quality management system and maintained throughout the entire lifecycle of the product.
In particular, the following laws, standards, and directives must be complied with:
- Medical Devices Act (“Medizinproduktegesetz”)
- Directive 93/42/EEC and MDR (Medical Device Regulation)
- Quality management according to DIN EN ISO 13485
- Risk management according to DIN EN ISO 14971
- Electrical safety DIN EN 60601–1
- Usability test according to DIN EN 60601–1‑6
- Software development according to DIN EN 60601–1‑4 and DIN EN 62304
SurgiTAIX AG offers an adapted consulting package especially for new companies and spin-offs. We offer all consulting services from a single source, starting with the development of a QM system, support in the creation of technical documentation and support during the approval process.
In addition to pure consulting services, we offer training courses and lectures on the following topics:
- Quality management for medical devices
- Risk management for medical devices
- Technical documentation
- Directive conformant development of electronics, software, and hardware
- Electrical safety
The highest quality and safety are at the forefront of our services. Benefit from our many years of experience in quality and risk management for your products!
CARAD risk management software
Risk management for medical devices
The DIN EN ISO 14971 standard applies to risk management and thus risk analysis specifically to medical devices. According to this, a risk management file must be created for each medical device in which the risk analysis including risk assessment and risk control, as well as information from the life cycle phases after delivery of the product are summarized and documented. It must be ensured that all responsible persons also have the necessary specialist knowledge and experience. This must also be documented in the risk management file.
The CARAD risk software
CARAD is software specially designed to simplify the risk management process. The input of risk analyzes with CARAD is process-oriented on the basis of the F ailure- M ode and E ffect — A nalysis (FMEA). At the same time, a corresponding fault tree analysis can be carried out at any time. Through the combination of FMEA and fault tree analysis, not only can the potential weak points of the system be easily identified, but their causes can also be easily assigned.
In order to increase the clarity, thematically related process steps are grouped into processes. Processes can be grouped together with further process steps to form higher-level processes, so that at the end a tree structure arises with processes as branches and process steps as leaves.
The form of the risk analysis report generated with CARAD corresponds to the FMEA form. For each error, all combinations of cause and consequence are determined and entered in a separate line of the table below the current work step with the corresponding risk figures. The risk numbers used to calculate the RPN (risk priority number) are dependent on the generic causes as well as on previous and subsequent errors.
The risk analysis report is output in PDF format.
CARAD runs on Windows 7, 8.x and 10.
CARAD is supported by the following Windows 10 editions: Windows 10 Pro and Windows 10 Enterprise.
Further information
Information on evaluation and comparison with other tools
More information about the CARAD software (download flyer)
Additional reading
As an introductory reading, we recommend the book by Prof. Dr. Jürgen P. Bläsing “Medizinprodukte: Risikomanagement im Lebenszyklusmodell”.
Download the software
Please write to us without obligation to: office [at] surgitaix.com. We will be happy to send you a free trial version.
Integration services
SurgiTAIX AG has been involved in the open, cross-manufacturer networking of medical devices for almost 15 years. In the research projects OrthoMIT and OR.NET, various standards for the networking of medical devices were defined and transferred to standardization. The result was the IEEE 11073 SDC family of standards, namely BICEPS, MDPWS and Architecture Binding. In the research projects ZiMT, MoVE, MOMENTUM and PriMed, application scenarios as well as approvability and test procedures were researched. The results of these research projects flow into the definition of the so-called Participant Key Purposes, which are currently in the ballotting phase of standardization. The PKPs define the requirements for devices depending on their function, for example, for devices that can be controlled externally or that issue or process alarms. Similarly, the so-called Device Specializations are currently being derived, in which the requirements for specific classes of devices such as RF devices, syringe pumps or operating tables are collected. The standardization was and is actively accompanied by SurgiTAIX AG.
sdcX — The commercial SDC library
In addition, SurgiTAIX AG has been active in the field of SDC libraries and software stacks for years. Such a stack enables a medical device manufacturer without in-depth knowledge of the standards to network his device via SDC. The stack takes on the role of a communication library and fulfills large parts of the requirements of the IEEE 11073 SDC family of standards. Previous, publicly available SDC stacks such as the SDCLib / C developed by SurgiTAIX ( https://github.com/surgitaix/sdclib ) and SDCLib / J ( https://bitbucket.org/surgitaix/sdclib ) were primarily designed for use in demonstrators and for technology evaluation. Use in commercial products was difficult due to the lack of documentation and risk assessment. The C ++ library sdcX, newly developed by SurgiTAIX, is the first stack that considers and fulfills the requirements for commercial operation. sdcX implements large parts of the three 11073 SDC core standards as well as parts of the PKP substandards that are currently under development.
The use of current technologies and programming methods enables high performance with moderate memory and resource consumption at the same time. The library can be used regardless of the mode of operation of the device or value-added service, as it supports different process models (callbacks, asynchronous programming, thread pooling).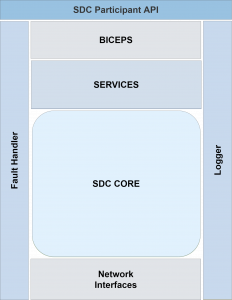 A focus during development was placed on the extensibility of the library — the X in the name stands for eXtensible. Through an extensible logger, logs generated by the library can be integrated directly into the user’s own device-specific loggers. Likewise own evaluations can take place by a multiplicity of subscribable notifications, for example with the entrance of a new message of a certain type. This allows the library to be used in various use cases and fields.
A focus during development was placed on the extensibility of the library — the X in the name stands for eXtensible. Through an extensible logger, logs generated by the library can be integrated directly into the user’s own device-specific loggers. Likewise own evaluations can take place by a multiplicity of subscribable notifications, for example with the entrance of a new message of a certain type. This allows the library to be used in various use cases and fields.
The library supports common ARM and x86 systems with Windows or Linux as operating system. Cross compilers for Buildroot or Yocto Linux can be integrated particularly quickly due to the CMake-based build process used. A comprehensive test suite is available on request for testing the library on the target platform. In this suite all parts of the standards and the library are tested and evaluated.
In addition to extensive documentation, a comprehensive risk analysis and IEC 62304-compliant development documentation are also provided.
Already during development, IT security requirements were determined and considered by a security process. This documentation of the process as well as the considered risks can be integrated into a documentation for the currently valid “FDA Premarket Submission for Management of Cybersecurity in Medical Devices”. The status of SOUP components will be continuously reviewed.
The requirements for medical devices are becoming more stringent, as are the demands of medical devices on the infrastructure. Especially missing consideration of real-time requirements as well as interfering cables complicate or prevent innovative use cases. To this end, the 5G-FORUM research project will expand the library to include support for real-time capable 5G networking with TSN. In the medium term, this will enable the library to be used in critical areas such as the control of control electronics, imaging or in robotics.
Interest piqued? Contact us for more information
